Lesson 4) Base Metals

Lesson 4 - Base Metals
Objectives:
- Learn how to identify base metals.
- Learn the importance of density.
We are officially at the halfway point of the year. Great job on getting this far! Remember that your midterm is today. Everything we have been over already as well as the information included in this lesson will be in the midterm. Don't worry, it won't be too difficult as long as you have studied. I would advise you to look over your notes and past tests one last time before taking the midterm.
It should not take you long to complete the test as well as review the questions before turning it in. Remember that the lessons should not be used on this exam, however you are allowed to use your own notes from the lessons.
This lesson will be a bit more difficult due to its extremely close ties with chemistry. We must get the basics out of the way before we get to discuss the magical aspect of this class. Now that we have established that, let’s talk about some base metals!
Remember back in the first lesson when we learned a bit about base metals? Base metals are elements that oxidize or corrode easily. Oxidation is when there is a loss of a subatomic particle (namely electrons) and an increase in the oxygen molecules in the metal. The opposite of this process is known as reduction (redox reaction).
Another way to tell if a metal is a base metal is to use a diluted form of hydrochloric acid. Hydrochloric acid is a colorless, odorless solution of hydrogen chloride and water that is found naturally in the stomach. Obviously if your body produces it to digest food then it's highly corrosive so remember to wear your dragonhide gloves while handling this acid.
This might sound dry and boring right now, but you will see that it's actually quite fascinating! As last week may have seemed as if you were stuck in a history class, this week we will take a trip to the more science side of things. Plus, who doesn't love messing with chemicals?
The first element we will discuss is lead.
Lead is a soft, malleable, yet heavy post-transition (elements located between transition metals and metalloids on the periodic table) metal that is a part of the carbon group. The scientific symbol for lead is Pb. Why is the symbol Pb when neither of those letters are in the word? It comes from the Latin word for lead, plumbum.
Lead is one of the easiest metals to manipulate with magic due to the amount of potential energy it carries. It has the right amount of energy that is prime for the transition into gold. It's known for its durability and resistance to change. Even Muggles use lead in firearms due to its good retention in velocity and energy.
When you are melting lead, you need to be aware of a few things:
- Melting point: 327.5° C / 621.5° F / 600.7 K
- Boiling point: 1740.0° C / 3164.0° F / 2013.2 K
- Density: 11.342 g/cm³
Around 2000 B.C.E., the people of ancient Egypt used lead to create coins. The Greeks saw that lead was less prone to corrosion than wood so they used a form of lead for the hulls of their ships.
As we learned last week, Dzou Yen was capable of transmuting lead into gold with a specific recipe that he concocted and no other alchemist seemed to be able to accomplish this again until Nicolas Flamel.
If you also remember the Harmony of Elements and Organs chart from last week's lesson, you will know that lead and Saturn are grouped together. It's said that some metals with an atomic weight greater than lead will disintegrate over time from radioactive decay and revert into lead. This is called nuclear transmutation and it's actually the reason why it's easier to transmute gold into lead instead of the other way around. This process is dictated by Saturn, which introduces the characteristic of time to the metals. Due to nuclear transmutation, lead is the lowest ranking base metal because metals revert into it, however, that doesn’t mean alchemists respect it any less. In alchemy, lead is the metal of transformation and redemption. We will talk more about time and Saturn at a later time.
The next element on our list is zinc.
Zinc is a bluish-white metal and the 24th most abundant element in the Earth's crust. The symbol for zinc on the periodic table is Zn. Paracelsus named zinc after the German word Zinke, though it's also known by the name Spelter. Zinc is known to be one of the weakest metals, especially pure zinc, which makes it very cheap.
- Melting Point: 419.5° C / 787.2° F / 692.7 K
- Boiling Point: 907.0° C / 1664.6° F / 1180.2 K
- Density: 7.134 g/cm³

Zinc is represented by five different symbols instead of just one. They all mean zinc, yet they don't look similar at all (with the exception of the third and fourth). Why are they all so different? It's simply due to location and time. These symbols vary depending on the country and time period a manuscript was written in. It actually isn't uncommon at all to have more than one symbol represent an element; especially considering how alchemy has been developed in different regions all over the world throughout time without a way for these different cultures to contact each other.
Zinc is most commonly found in the form of an alloy. An alloy is a mixture composed of an element and a metal. You all have heard of brass right? Of course you have! You bought brass scales when you were a first year because it was on the supply list. Brass is an alloy of zinc and copper that has been used at least as far back as the 10th century B.C.E. in Judea.
Alchemists burned zinc in the air as part of their rituals. This would leave a white wooly substance called philosopher's wool or nix alba. Nix alba is Latin for "white snow." In scientific terms, this substance is commonly known as zinc oxide (ZnO). It has many uses such as pigments in paints and in ointments to treat skin diseases.
Zinc is an important mineral in the body if you want to keep your health in a relatively good condition. Zinc deficiency is actually linked to several diseases that affect at least two billion people. However, taking too much zinc is also bad for you because that can lead to other problems such as lethargy and copper deficiency.
Up next is nickel.
Nickel is a silvery-white element with a golden tinge that has a high polish. The symbol for nickel on the periodic table is Ni. It's hard and ductile. Nickel is actually considered corrosion-resistant due to its slow rate of oxidation at room temperature. Larger pieces of nickel are slower to react with air because they form a protective oxide surface. Even then the metal is still reactive enough that it is more commonly found within Earth's crust rather than the surface. It's one of the four metals magnetic at room temperature; the other three are iron, cobalt, and gadolinium.
- Melting Point: 1453.0° C / 2647.4° F / 1726.2 K
- Boiling Point: 2732.0° C / 4949.6° F / 3005.2 K
- Density: 8.902 g/cm³
Nickel has been used as far back as 3500 B.C.E. in the form of alloys used to make Syrian bronzes. It was also used to make Chinese coins in 235 B.C.E. It was classified as an element and isolated in 1751 by Axel Fredrik Cronstedt. Cronstedt was attempting to extract copper out of a mineral called niccolite, however, instead of copper he ended up with the white metal we now know as nickel. It's named after a mischievous sprite from German miner mythology named Nickel. Cronstedt was also one of the founders of modern mineralogy, discovered scheelite, and named the element tungsten.
Nickel can be used as a green tint in glass depending upon its concentration, and is also capable of producing blue, violet, and black glass. Witches and wizards use the Mineral Concentration Charm to change the color of glass. This works by transfiguring the amount of a mineral in the glass that is used to give it its color.
Spell Practice:
Name: Mineral Concentration Charm
Incantation: Morbi videntur (mor-BEE vi-DEN-ture)
Wand Movement: Starting from the left, drag your wand down diagonally to the right and then bring it back up diagonally continuing towards the right. You should end up with a V-shape. After drawing the V, make a light jab with your wand towards the object.
This spell works by focusing on which color you want the glass to change to and the concentration of the mineral creating the color in the glass. Of course, this spell works better if you know which minerals are contained in the target glass to begin with. When the spell is done correctly, the glass will instantly change colors. If the spell is done incorrectly, a small crack will form down the middle of the glass.
For this exercise, I have provided everyone with a piece of glass on their table. Each piece of glass has a small concentration of nickel, but not enough to give it a color yet. I want you to decide what color you would like it to be and adjust the concentration depending on the color you choose. For example, a little bit more nickel would turn the glass green while transfiguring as much nickel as possible into the glass will turn it black. If it's not the color you were expecting, don't worry! As long as you perform the spell correctly, you can keep changing the colors as much as you like. If you perform the spell incorrectly and your glass cracks, let me know and I will provide you with another piece of glass.
The last base metal we will talk about today is iron.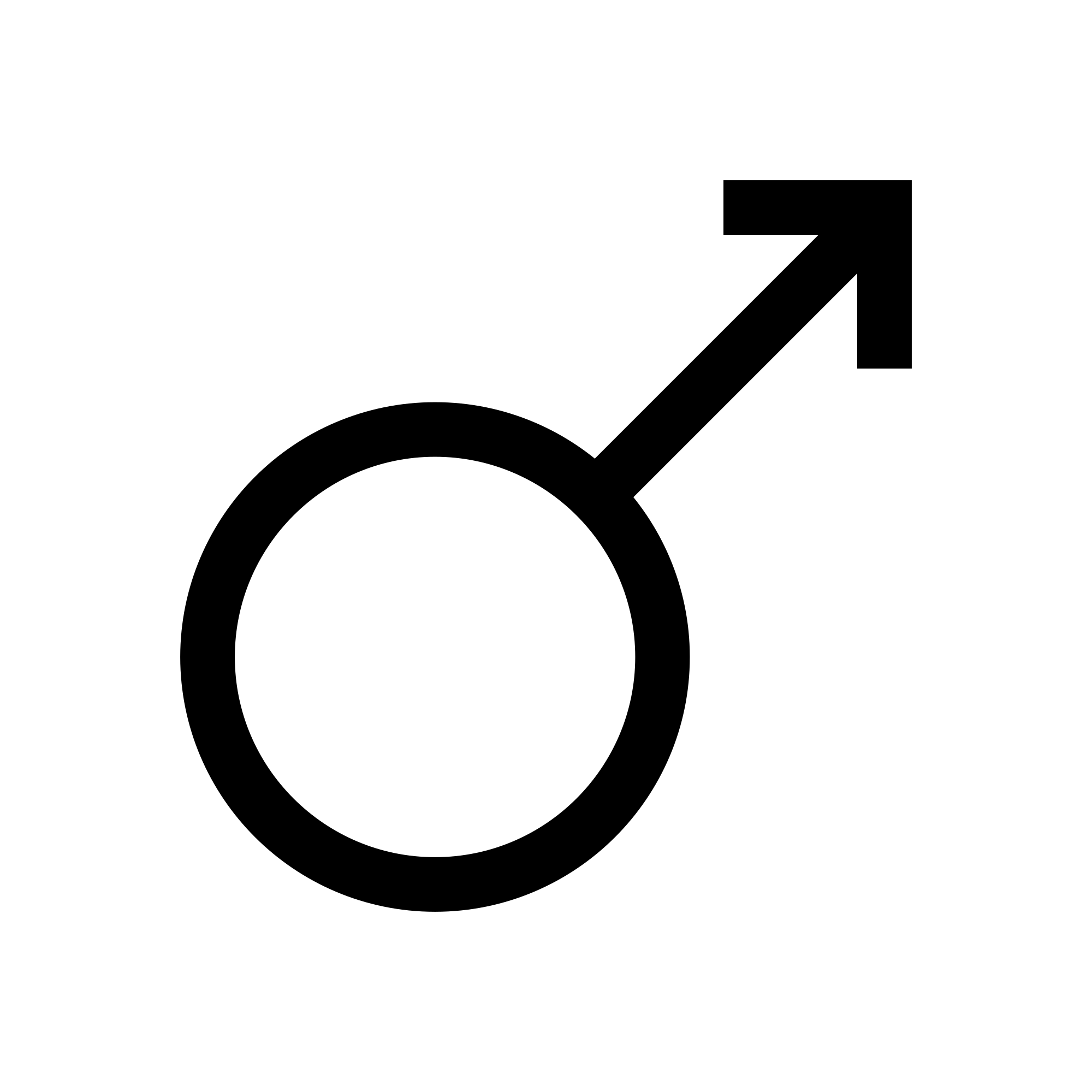
Iron is a lustrous silvery-gray metal. Its symbol on the periodic table is Fe. As mentioned earlier, iron is one of the four metals that are magnetic at room temperature. It's the most common element found on Earth by mass and it's the fourth most abundant element in the Earth's crust.
- Melting Point: 1535.0° C / 2795.0° F / 1808.2 K
- Boiling Point: 2750.0° C / 4982.0° F / 3023.2 K
- Density: 7.86 g/cm³
When iron is met with oxygen in the presence of water or moisture in the air, it oxidizes and forms iron oxides. You may all know this as rust. Rust develops on the outside of the metal and occupies more volume than the metal. Due to this, it flakes off and exposes the fresh iron underneath to go through the whole process again.
Like zinc, iron is very important in maintaining good health. Every living organism has iron-proteins in their body. Have you ever bit your lip and wondered why your blood has a metallic taste? The color of your blood is because of hemoglobins, which are proteins that contain iron. Other enzymes and proteins that contain iron mainly do biological oxidations and transportation.
Iron was also listed on the Harmony of Elements and Organs chart created by Paracelsus. He linked iron up with Mars, and rightly so I might add. The soil on Mars is high in concentration of iron and most of it is oxidized as that's what gives the planet its red color. Mars is often associated with blood, war, and fire. Out of the seven metals listed on the chart, iron is the only metal that burns. These similarities aren't subtle in the least. The symbol for iron is associated with the male gender; therefore, iron is considered to be very masculine.
Why is Density important?
 The last thing we are going to talk about today is something all of us have: density. Density is one of the most important characteristics in the universe as it gives us a way to relate mass and volume. You can determine the density of an object by dividing the mass of it by the volume.
The last thing we are going to talk about today is something all of us have: density. Density is one of the most important characteristics in the universe as it gives us a way to relate mass and volume. You can determine the density of an object by dividing the mass of it by the volume.
 Density can be increased or decreased depending on how you affect the mass and/or volume of an object. For example, let's say you have two balloons of the same mass. You take one balloon and try to compress it into a small size. By squeezing the balloon, you are decreasing its volume thus causing the balloon to become denser due to the air particles inside having less room to move. Another example of increasing the density would be increasing the mass of the object while keeping the volume constant. Let's say you have a bowling ball and a volleyball that are both the same size. Which is heavier? Obviously the bowling ball will be much heavier because it has more mass than the volleyball, thus the bowling ball is the denser one of the two.
Density can be increased or decreased depending on how you affect the mass and/or volume of an object. For example, let's say you have two balloons of the same mass. You take one balloon and try to compress it into a small size. By squeezing the balloon, you are decreasing its volume thus causing the balloon to become denser due to the air particles inside having less room to move. Another example of increasing the density would be increasing the mass of the object while keeping the volume constant. Let's say you have a bowling ball and a volleyball that are both the same size. Which is heavier? Obviously the bowling ball will be much heavier because it has more mass than the volleyball, thus the bowling ball is the denser one of the two.
The reason you float in water is credited to density. In order to float, you have to have a smaller density than what substance you are floating in. Have you ever tried to mix oil and water together? They don't really mix and you will notice that the oil will always move above the water. That's because oil is less dense than water. A density tower like the one shown is a fascinating way of showing how this works.
Now that we've dealt with some science, it's time for your midterm. I also have an essay for those of you who want to write about your experience with the Mineral Concentration Charm for extra house points. I would advise you to write your essay after you hand in your midterm, however. You can take this time to look back over past lessons, tests, and notes before starting. The midterm is not difficult at all and you can use your own notes on it.
Enroll
-
Midterm Exam
Test -
Staining Glass
Essay
-
Elizabeth Schulte
Head Student
-
Claire Peters
Professor's Assistant
-
Sammy Morse
Professor's Assistant
-
Isobel Watford
Professor's Assistant


